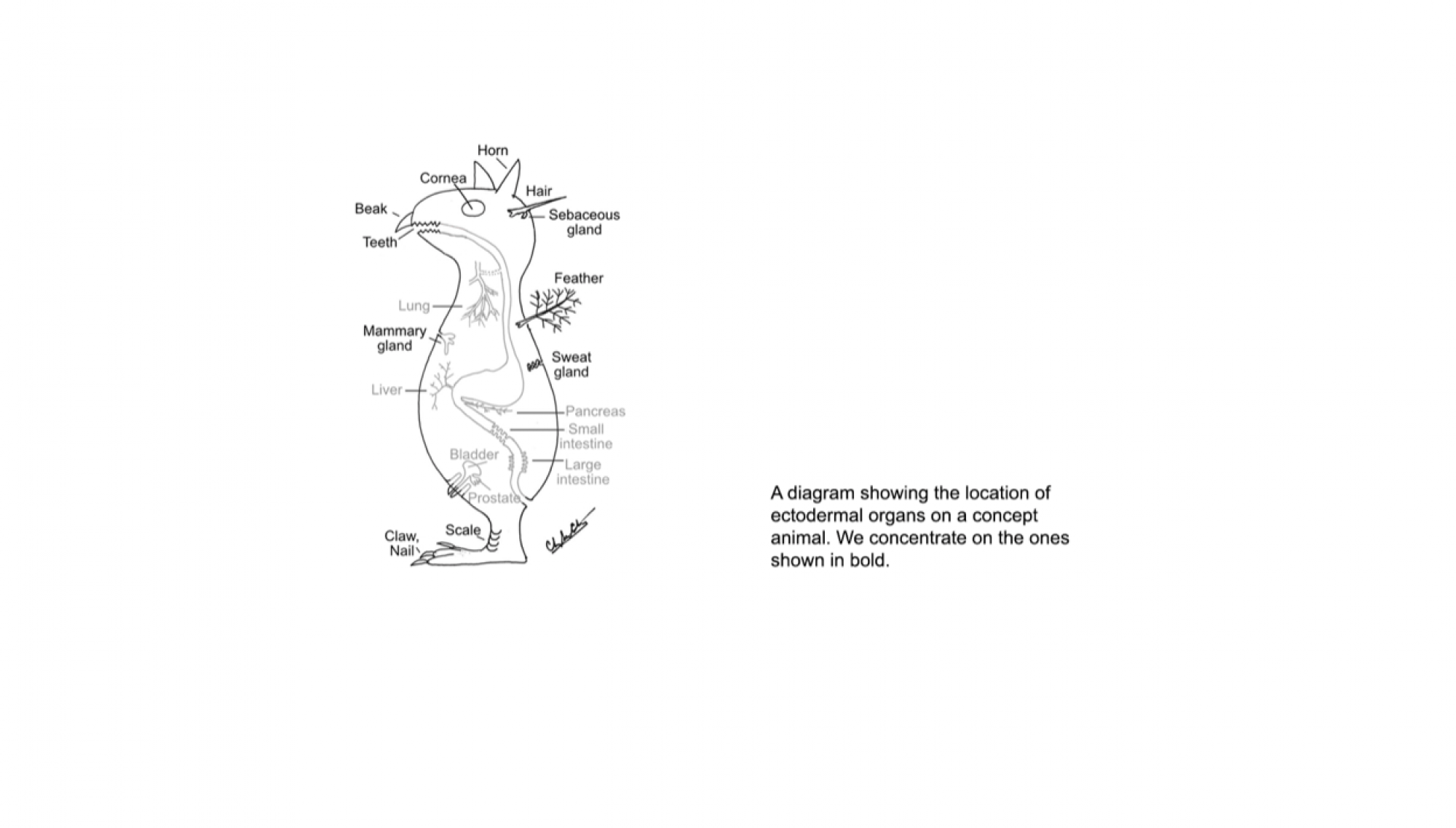
The laboratory asks fundamental questions in bio-medical research. The focus of research is morphogenesis, i.e. how cells are assembled into functional forms. We are concerned with the principles that determine the specific number, size and shape of different organs. This is an important process, as there is much to learn about the principles that can guide stem cells to form specific tissues and organs required for medical treatment. Our approach is to ask Nature how she does it – using the feather as a Rosetta stone to decipher these principles, because of the distinctive forms of feathers and interest in the evolution of flight. This unique model has allowed us to make several major impacts in the field of morphogenesis and develop interesting interfaces with scientists in different disciplines.
Representative papers
a. Jiang, T.-X., Jung, H.S., Widelitz, R.B., Chuong, C.-M. 1999. Self-organization of periodic patterns by dissociated feather mesenchymal cells and the regulation of size, number and spacing of primordia. Development 126:4997-5009. (An early landmark paper that integrates the Turing model with self-organizing behavior of periodically arranged feather buds from dissociated single cells).
b. Yu, M., Wu, P., Widelitz, R.B., and Chuong, C.-M. 2002. The Morphogenesis of feathers. Nature 420:308-312. (A paper that established a new model to study the regenerating feathers, which triggered new understanding of the evolution of protofeathers in feathered dinosaurs).
c. Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R and Chuong CM. 2008. Cyclic dermal BMP signaling regulates stem cell activation during hair regeneration. Nature 451:340-344. (A paper that studied hair cycling in living mice and found dermal BMP cycling in the intradermal adipose tissue, modulating the rhythm of hair cycling. It catalyzed the search for more examples of macro-environmental modulation of stem cells. Cited 574 times).
d. Chang WL, Wu H, Chiu YK, Wang S, Jiang TX, Luo ZL, Lin YC, Li A, Hsu JT, Huang, HL, Gu HJ, Lin TY, Yang SM, Lee TT, Lai YC, Lei M, Shie MY, Yao CT, Chen YW, Tsai, JC, Shieh SJ, Hwu YK, Cheng HC, Tang PC, Hung SC, Chen CF, Habib M, Widelitz RB, Wu P, Juan WT, Chuong CM. 2019. The Making of a Flight Feather: Bio-architectural Principles and Adaptation. Cell. 179:1409-1423. (This is a paper produced by a multi-disciplinary team involving work from developmental biologists, biophysicists, and ecologists that were organized to unravel new understanding on flight feather architecture. This study inspires biomimetic biomaterial designs and was featured as the cover of that issue).
Contributions to Science
1. Distill organizing principles of integumentary organs. In the 1990s, when Shh, Wnt, FGF morphogen growth factors and Hox, Msx, and other transcription factors were found to be involved in organ formation, I was among the pioneers to apply them to study skin development. In addition to the research articles mentioned above, in 1998, I edited a book on “Epithelial Appendage Morphogenesis: Variations of a common theme and implications in regeneration”. In 2003, I edited another special issue on Evo-Devo of Integument and in 2009 on Pattern Formation. Since then, I was frequently invited to write commentary pieces for Science (The Tao of integuments, 2016; Aging, alopecia and stem cells, 2016) ), Nature (New hairs from wounds, 2008), Cell Stem Cells (The river of stem cells, 2009; Epidermal Darwinism, 2018), Plos Biol (Turing with and without global wave), etc. to highlight new concepts in the field. The integrations helped to inspire more investigators to enter the field. In addition, my team took on multi-disciplinary collaborations with mathematicians, biophysicists, electrophysiologists, paleontologists, evolutionary biologists, robot scientists, to largely expand the scope of skin biology. The Shen et al., paper was with a robot engineer and has been cited widely by those who study robot swarming behavior. The Chang et al., Cell cover paper (2019) was praised by those searching for bio-inspired biomaterial design. The Li et al., Nat Comm paper (2018) connected us with bioelectricity investigators. Thus, our contribution has achieved a holistic understanding in the development and regeneration of skin appendages, which has enabled us to reach a wide audience and to expand these fields.
a. Chuong CM edit, 1998. Molecular Basis of Epithelial Appendage Morphogenesis: Variations of a common theme and implications in regeneration. A book published by Landes Biosciences. Austin.
b. Chuong, CM. and Homberger, D.G. edit, 2003. Development and Evolution of the Amniote Integument. A special issue for Molecular Developmental Evolution, J Exp Zool. 298B.
c. Chuong, CM and Richardson, M edit. 2009. Pattern formation. Int. J. Dev. Biol. Vol 53. (A special issue on pattern formation).
d. Lai, Y. C., Chuong, C. M. 2016. The Tao of integuments. Science. 354:1533-1534. (This is presented as a short perspective. A full review is in process).
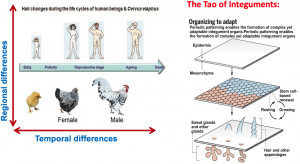
Contribution 1: Distill organization principles of skin appendages. Dr. Chuong has the spirit of a basic biologist. While he seems to work on the integuments of diverse animals, he believes they are guided by only a few simple fundamental principles. The left panel show the changing skin appendage patterns under physiological regeneration in different ages (Chuong et al., 2012, Physiology). While humans and primates have complex facial skin patterns, mice do not. So, he almost obsessively dedicates his research to the bird model with the hope to identify these rules. His NIH dermatology colleagues appreciate the contributions of his work and honored him with the Society’s highest Kligman/Frost award. In the “Tao of the Integument”, he states, Principle 1, periodic patterning, producing multiple elements. Principle 2, each element obtains stem cells for cyclic renewal. Principle 3, the power of multiplicity builds upon diversification in a temporo-spatial way to optimize the integument function at different ages and regions of the same organism. Principle 4, These changes are integrated in evolution for adaptation to new eco-spaces. (Lai and Chuong, Science, 2016).
2. Elucidate the tissue patterning process in the developing skin. How cells are organized into tissue architectures is a fundamental question that is important to development and regeneration. In the 1990s, although Turing reaction-diffusion had been proposed to play a role in tissue patterning, identification of specific activators/inhibitors in biological patterning had lagged behind. Originally, I worked with Dr. Wolpert to show FGF/BMP fulfills the criteria in feather pattern formation and later developed mathematical modeling with Dr. Maini of Oxford University. The combined experimental data / model approach inspired more scientists to work out periodic patterning in the integuments they worked on. While diffusible morphogens are important, more recent works imply that biophysical forces are also involved, including bio-electrical and bio-mechanical forces which can transmit signals faster and over longer distances than molecular signals to coordinate collective cell movements. My team also work on this this new frontier.
a. Jung, H. S., Francis-West, P. H., Widelitz, R. B., Jiang, T. X., Ting-Berreth, S., Tickle, C., Wolpert, L., Chuong, C. M. (1998). Local inhibitory action of Bmps and their relationships with activators in feather formation: Implications for periodic patterning. Dev Biol, 196: 11-23. (The first paper to show FGF, BMP work as Turing activators / inhibitors in skin appendage formation. Cited 373 times).
b. Jiang, T.-X., Jung, H.S., Widelitz, R.B., Chuong, C.-M. 1999. Self-organization of periodic patterns by dissociated feather mesenchymal cells and the regulation of size, number and spacing of primordia. Development 126:4997-5009. (A landmark paper that integrates the Turing model with self- organizing behavior of periodically arranged feather buds from dissociated single cells).
c. Li A, Cho JH, Reid B, Tseng CC, He L, Tan P, Yeh CY, Wu P, Li Y, Widelitz RB, Zhou Y, Zhao M, Chow RH, Chuong CM. 2018 Calcium oscillations coordinate feather mesenchymal cell movement by SHH dependent modulation of gap junction networks. Nat Commun. 9:5377 (This paper reveals new integration of bio-electric signals with biochemical signals during feather elongation).
d. Wu XS, Yeh CY, Harn HI, Jiang TX, Wu P, Widelitz RB, Baker RE, Chuong CM, 2019. Self-assembly of biological networks via adaptive patterning revealed by avian intra-dermal muscle network formation. Proc Natl Acad Sci U S A. 116:10858-10867. (A paper showing the novel concept of how dermal muscle networks self-organize using feather follicles as nodal points, and adapt to changes in the skin architecture due to damage).
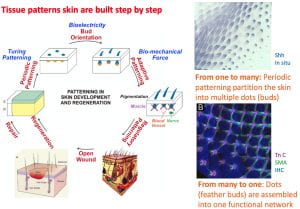
Contribution 2: Tissue patterning in the developing skin. Beginning by working on the de novo periodic patterning of hexagonally packed feather buds, Dr. Chuong continued to analyze how different layers are assembled step by step in 1) de novo periodic patterning, 2) adaptive patterning and 3) regulatory patterning. His team identified how biochemical and biophysical forces work together to build up complex tissue patterns. This long line of research won his team an NIH Merit award in 2020. His team now continues to investigate these self-organization processes.
3. Studying how region-specific feathers are built. One of the fundamental questions remains to be answered is how region-specific skin and appendages form. The feather, with distinct morphology, is an excellent model as they morph from chick downy feathers into flight, tail, or contour feathers in the adult. Starting in the 2000s, my team set up the novel regenerating feather / viral mediated gene mis-expression model which allows us to identify molecular circuits involved in forming radially symmetric, bilaterally symmetric, and bilaterally asymmetric feathers, as well as barb branch types (feather vane vs fluffy branches) from the proximal to distal feather. In addition to feather forms, we study region-specific keratins and pigmentation patterns. We are the first to identify both feather follicle epidermal stem cells and melanocyte stem cells. We showed how they are molded into specific forms and patterns depending on the niche topology and molecular signals they encounter when they are generated. Furthermore, we take an epigenetic approach to study how these regional specificities are established. We found beta-keratins clustered in Chromosome 27 form high order temporal-spatial specific chromatin loops to generate a large spectrum of beta-keratins with different rigidities needed to build diverse feather architectures. The work also uncovered a novel strategy for gene cluster regulation. We are currently working on how epigenetic memory is stored in the feather dermal papilla. Thus, my team has taken the lead by establishing this new model system to unravel the molecular control of region-specific skin appendage morphology in development, regeneration and adaptation.
a. Yue, Z. C., Jiang, T. X., Widelitz, R. B., Chuong, C. M. 2005. Mapping stem cell activities in the feather follicle. Nature, 438: 1026-1029. (It shows the follicle bulge is the site for feather follicle stem cells).
b. Lin SJ, Foley J, Jiang TX, Yeh CY, Wu P, Foley A, Yeh CM, Huang YC, Cheng HC, Chen CF, Reeder B, Jee SH, Widelitz RB, Chuong CM. 2013. Topology of feather melanocyte progenitor niche allows complex pigment patterns to emerge. Science. 340:1442-1445. (The paper identifies follicle melanocyte stem cells and shows the multiple mechanisms used to generate intra-feather pigment patterns, and also the influence by sex hormones).
c. Li A, Figueroa S, Jiang T-X, Wu P, Tang P-C, Chen C-F, Widelitz R, Nie Q, Chuong C-M. 2017. Diverse feather shape evolution enabled by coupling anisotropic signaling modules with self-organizing branching program. Nature Commun 14139. (The paper sums up the molecular controls of feather forms: radially symmetric downy, bilaterally symmetric contour and bilaterally asymmetric flight feathers by BMP, Wnt and RA pathways).
d. Liang YC, Wu P, Lin GW, Chen CK, Yeh CY, Tsai S, Yan J, Jiang TX, Lai YC, Huang D, Cai M, Choi R, Widelitz RB, Lu W, Chuong CM. 2020. Folding keratin gene clusters during skin regional specification. Dev Cell. 53:561-576. (The work breaks new ground on how the high order chromatin configuration of beta keratin (or CBP, an EDC member) generates a large spectrum of complexity).
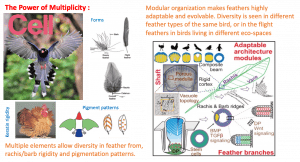
Contribution 3: How are region-specific feathers built from the same genome. As illustrated in Cell cover (2019, Nov), they analyze diverse skin appendages on the same bird in terms of feather forms (radial, bilateral symmetry, bilateral asymmetry), pigmentation patterns (Macro- for across the body patterns, and micro- for within-feather patterns), and branch hardness. The Frizzled chicken with a keratin mutation provided a clue for them to discover a novel high order chromatin looping of beta keratin gene clusters (Liang et al., Developmental Cell, 2020). They use regenerating feather follicle model to identified both epidermal and pigment stem cells. In a series of papers, his team demonstrated how Wnt, BMP, Shh, signaling work together in a topological- and temporo-specific manner to sculpt out the feather forms and paint them with unique color patterns. The right panel shows how rachis cortex/ medulla architectures inspires bio-composite design, and how barbs are fluffy or form vane with hooklets (Chang et al., Cell, 2019). Now they move on to study how the epigenetic memories are kept in different body regions.
4. Reveal collective regenerative behavior in a hair follicle population. Many studies on hair stem cells focus on the cyclic regeneration of a single hair follicle. However, each individual animal has thousands of hair follicles and the collective regeneration behavior of the hair follicle population has not been well-studied. In 2008, we demonstrated intra-dermal adipose tissue exhibits BMP cycling which is out of phase with epidermal beta-catenin cycling in hair follicles. Further, the intra-dermal adipose BMP remains high during pregnancy and lactation to maintain hairs for nursing. These findings led us to promote the concept that the extra-follicular macro-environment (hormones, seasons, aging, etc.) also regulates hair follicle stem cell activity. This line of work has inspired many to explore more different macro-environmental modulations of hair follicle stem cells in subsequent years. Among them is our own work showing the coupling of immune signaling with stem cell activation after hair plucking. With topologically well-positioned plucking, we can pluck 200 hairs to get up to 1000 hairs activated in a quorum sensing fashion. Another angle is on wound induced follicle neogenesis. Our experience in periodic patterning helps us modulate macro environments that facilitate epidermal and dermal cells to form a morphogenetic field competent of periodic patterning.
a. Plikus, M.V., Baker, R.E., Chen-C.C., Fare, C., de la Cruz, D., Andl, T., Maini, P.K., Millar, S., Widelitz, R. and Chuong, C.M. 2011. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science 332:586-589. (It shows amazing fractal type regenerative hair wave spreading).
b. Chen, C-C., Wang, L., Plikus, M.V., Jiang, T.X., Murray, P.J., Ramos, R., Guerrero-Juarez, C.F., Hughes, M.W., Lee, O.K., Shi, S., Widelitz, R.B., Lander, A.D., Chuong, C.M. 2015. Organ-level quorum sensing directs regeneration in hair stem cell populations Cell. 161:277-290. PMID: 25860610; PMCID: PMC4393531. (A paper that integrates regeneration, immune mechanism, and organ-level collective behavior, presented with mathematical modeling. We got a lot of coverage in popular science magazines and inquiries from many volunteers).
c. Lei, M., Schumacher, L.J., Lai, Y-C., Juan, W-T., Yeh, C-Y., Wu, P., Jiang, T-X., Baker, R.E., Widelitz, R.B., Yang, L., Chuong C-M. 2017. Self-organization process in newborn skin organoid formation inspires novel strategy to restore hair regeneration of adult cells. Proc Natl Acad Sci U S A. (A paper demonstrating how dissociated epidermal and dermal cells can reorganize into reconstituted skin with properly arranged hair follicles. A remarkable model for self-organization study and regenerative engineering).
d. Harn HI, Wang SP, Lai YC, Van Handel B, Liang YC, Tsai S, Schiessl IM, Sarkar A, Xi H, Hughes M, Kaemmer S, Tang MJ, Peti-Peterdi J, Pyle AD, Woolley TE, Evseenko D, Jiang TX, Chuong CM. Symmetry breaking of tissue mechanics in wound induced hair follicle regeneration of laboratory and spiny mice. Nat Commun. 2021 May 10;12(1):2595.
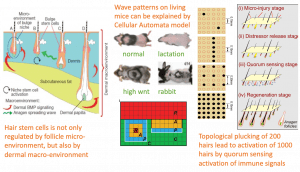
Contribution 4: Demonstrate the collective regenerative behavior in a hair follicle population which opens new application possibilities. With thousands of hair follicles on the body surface of the mouse, they developed new ways to observe regenerative hair wave rhythms in living mice that spread in days to weeks. The rhythms can be altered by pregnancy (pausing hair loss so they can provide warmth for the litter), aging (slower), high wnt levels (fast wave), or superfast fractal like spread (rabbit, with multiple stem cells in one follicle). In this case, they show the cyclic BMP-based rhythm in dermal adipocytes modulate beta catenin-based hair cycling rhythm. He collaborates with Dr. Maini / Baker to use Cellular Automata which can explain and predict these behaviors. (Plikus et al., 2008, Nature, 2011, Science). The concept expanded to imply additional macro-environmental layer of modulation. They went on to show a well-planned hair plucking can induce immune signaling which are coupled with large scale regeneration. A quorum sensing model was developed with Dr. A. Lander (Chen et al., Cell, 2015).
5. Provide new insights in the Evo-Devo of integumentary organs. New fossil findings in the last three decades provide evidence of feathered dinosaurs which stimulated the imaginations of scientists and the general public. Yet the morphologies of these protofeathers are unusual. Our experimental data help to make sense of the evolution of feathers. In addition to the micro-evolution (organ shape, size) of one integumentary organ (e.g., feather, tooth, beak), we also worked out potential macro-evolution mechanisms by converting one integument type into the other (e.g., scales vs feathers). Furthermore, we demonstrated the regenerative mechanism of three follicle types (hair, feather, and tooth) are based on different stem cell activation mechanisms based on convergent evolution. Thus, our interface with paleontologists and evolutionary biologists have successfully enriched each other’s fields to achieve a more holistic understanding.
a. Wu, P., Jiang, T. X., Suksaweang, S., Widelitz, R. B., Chuong, C. M. (2004). Molecular shaping of the beak. Science, 305:1465-1466. (It shows BMP localized growth zone can shape the beak, cited 251 times).
b. Wu P, Wu XS, Jiang TX, Chen MH, Elsey R, Temple BL, Hernandez-Divers SJ, Yuan K, Travis G, Chen MH, Widelitz RB, Chuong CM. 2013. Specialized stem cell niche enables repetitive renewal of alligator teeth. Proc. Natl. Acad. Sci. U S A. 110:2009-2018. (A unique paper demonstrating how alligators renew multiple tooth generations by having 3 teeth in a single tooth family and control stem cell activation via SFRP1 niche regulation).
c. Wu P, Yan J, Lai YC, Ng CS, Li A, Jiang X, Elsey R, Widelitz R, Bajpai R, Li WH, Chuong CM. Multiple regulatory modules are required for scale-to-feather conversion. Mol Biol Evol. 2018 35(2):417-430. (Omics study to identify differential expression between feathers/scales, with functional verifications).
d. Xu X; Zhou Z, Dudley R, Mackem S, Chuong CM, Erickson GM, Varricchio DJ, 2014, An integrative Approach to Understanding Bird Origins. Science, 346: 1253293. (A review. It contributes to the choice of “The birth of birds” by Science as one of the 10 breakthroughs in 2014. Cited 189 times).

Contribution 5: Evo-Devo of feathers, scales, and other integuments. Fascinating fossil finds have provided evidences of feathered dinosaurs and stimulated the imagination on how feathers evolved. The multi-disciplinary collaboration with paleontologists led to the choice of “The Feathers of Birds” as one of the top 10 breakthroughs selected by Science in 2014 (Xu et al., 2014, Science). Amber fossil: Wang et al., 2020, Evolution). Using systems biology analyses, they identify multiple morpho-regulatory modules that covert scales into feathers through both combinatorial and hierarchical integration (Wu et al., 2017, Molecular Biology Evolution). They also work on beak shaping by analyzing chicken, duck and cocktail. They also work out the control of alligator tooth cycling.