Overview
Cellular membranes provide critical interfaces for the biochemical interactions that regulate cell behavior and function. These membranes are composed of a heterogeneous mixture of lipids and proteins. Understanding and controlling biomolecular interactions that occur at membranes is challenging owing to the inherent ability of the membrane environment to functionally couple interactions between proteins and lipids. Nonetheless, elucidating the interplay between proteins and lipids in biological membranes is important for a variety of applications, such as pinpointing the mechanisms of disease, discovering and delivering new drugs, and developing functional biomaterials. Toward addressing these challenges, our group uses engineering principles and fluorescence techniques to develop the tools, approaches, and ideas that will enable us to understand and control the protein-lipid interface.
Intrinsically Disordered Proteins at Curved Biological Membranes
Lipid bilayers are the primary structural component of cellular membranes and are resistant to deformation. However, curvature is ubiquitous in the cell as membrane trafficking and organelle biogenesis are essential to cellular function. Cells must utilize proteins that can sense local curvature to facilitate processes that are necessary for cellular physiology, such as cell signaling, exocytosis, and endocytosis. Interestingly, intrinsically disordered proteins (IDPs) comprise a significant fraction of the human proteome and are heavily implicated in membrane trafficking.
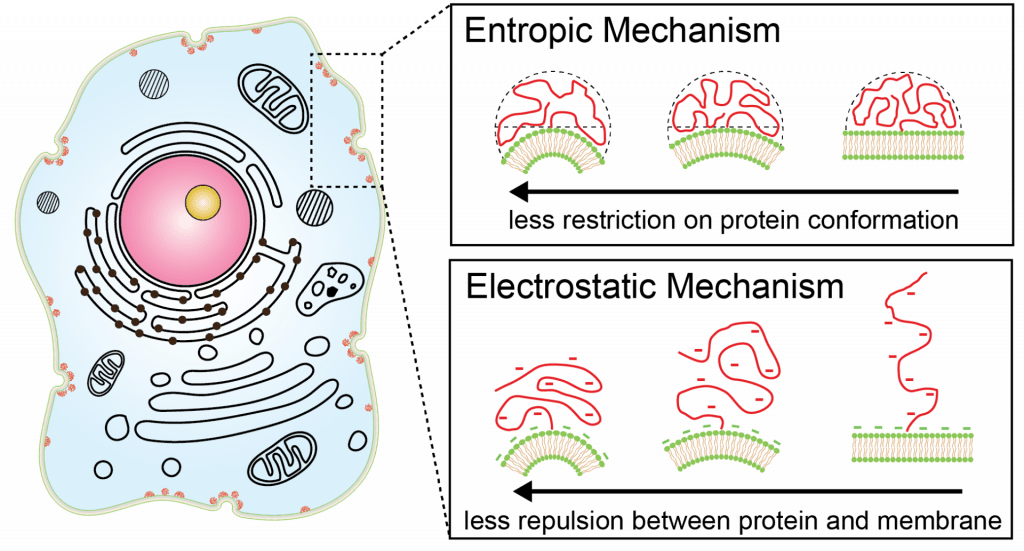
IDPs are polymer-like proteins that lack substantial secondary structure. They instead take on an ensemble of conformations, which can be characterized by a radius of gyration. This dynamic behavior elicits interesting biophysical behavior when IDPs are brought in close proximity to membrane surfaces. Previous research by Zeno et al. has revealed that IDPs are potent sensors of membrane curvature, despite their lack of structure. These findings represent a substantial departure from the structure-function paradigm, which posits that function only arises from proteins with structure. Two biophysical mechanisms of curvature sensing by IDPs are illustrated above. With the entropic mechanism, IDPs preferentially partition to highly curved surfaces due to minimal steric hindrance and impact on their conformational entropy. The electrostatic mechanism drives negatively charged IDPs to highly curved surfaces as this geometry minimizes coulombic repulsion with anionic membrane surfaces. Together, these two mechanisms synergistically make IDPs potent and robust sensors of membrane curvature.
 Alpha-synuclein is a neuronal IDP that interacts with synaptic vesicles. It is a biologically significant protein, as its malfunction is implicated in the onset of Parkinson’s Disease. As shown above, alpha-synuclein adopts a partial helical structure upon interaction with the membrane surface of synaptic vesicles. However, it maintains a substantial region of disorder in close proximity to the membrane. Part of our ongoing work is understanding how the structural features of this protein influence its interaction with biological membranes.
Alpha-synuclein is a neuronal IDP that interacts with synaptic vesicles. It is a biologically significant protein, as its malfunction is implicated in the onset of Parkinson’s Disease. As shown above, alpha-synuclein adopts a partial helical structure upon interaction with the membrane surface of synaptic vesicles. However, it maintains a substantial region of disorder in close proximity to the membrane. Part of our ongoing work is understanding how the structural features of this protein influence its interaction with biological membranes.
Graphene Quantum Dots for Therapeutic Applications
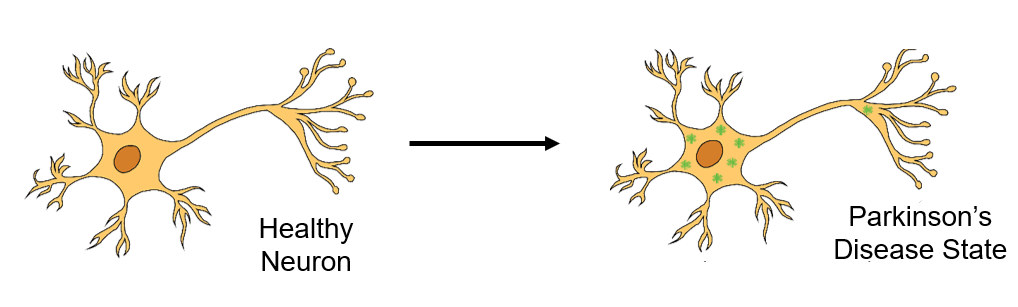 Malfunctioning alpha-synuclein forms neuronal aggregates that are disruptive to neurotransmission and neuronal health. These aggregates also induce oxidative stress in the mitochondria. Recent work from collaborators revealed that Graphene Quantum Dots (GQDs) can inhibit aggregation of alpha-synuclein and mitigate oxidative stress in mitochondria. However, the mechanism of action for these GQDs is poorly understood.
Malfunctioning alpha-synuclein forms neuronal aggregates that are disruptive to neurotransmission and neuronal health. These aggregates also induce oxidative stress in the mitochondria. Recent work from collaborators revealed that Graphene Quantum Dots (GQDs) can inhibit aggregation of alpha-synuclein and mitigate oxidative stress in mitochondria. However, the mechanism of action for these GQDs is poorly understood.
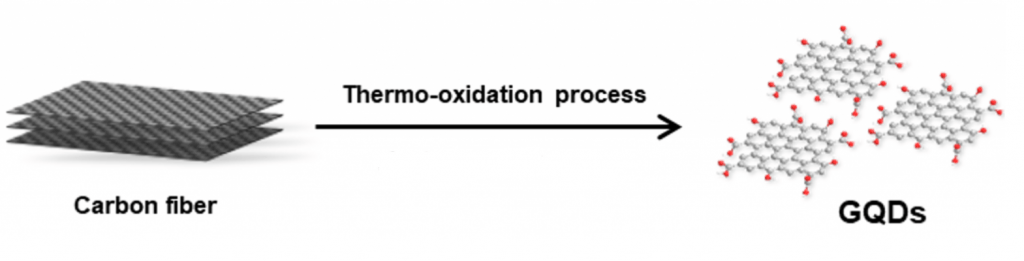 Part of our ongoing work is to develop a mechanistic understanding of the interactions between GQDs and biomolecular systems. These findings will help toward the development of GQDs as a therapeutic for neurodegenerative and lysosomal diseases.
Part of our ongoing work is to develop a mechanistic understanding of the interactions between GQDs and biomolecular systems. These findings will help toward the development of GQDs as a therapeutic for neurodegenerative and lysosomal diseases.
Membrane-Inspired Biomaterials
The plasma membrane is a natural barrier that allows for selective permeation of biomolecules. Therefore, it is important to understand the mechanisms by which cells internalize and traffic cargo. Through this understanding, we can develop materials that can effectively cross the plasma membrane to deliver therapeutics to the cell. Recently, this research area has been particularly relevant in the field of soft matter biophysics. This relevance is evidenced through the development of liposomal-based vaccines that were administered by Moderna and Pfizer. In our research group, we are aiming to develop nanodiscs as therapeutic delivery vehicles.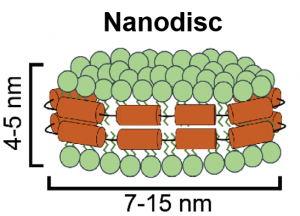 Nanodiscs are discoidal patches of lipid bilayers that have their hydrophobic region shielded by amphipathic scaffolding proteins. Their nanoscale dimensions make them quite amenable for cellular uptake, while their ability to be functionalized with peptides and nucleic acids make them very appealing to drug delivery applications. Part of our ongoing work is synthesis and characterization of these particles, as well as engineering them and assessing their delivery to live cells. Nanodiscs can also incorporate integral membrane proteins. Therefore, we are also interested in developing functional biomaterials, such as immobilized protein substrates. These types of substrates can be used as diagnostic devices for drug screening and perhaps even have applications in Adoptive T Cell Therapy for cancer treatment.
Nanodiscs are discoidal patches of lipid bilayers that have their hydrophobic region shielded by amphipathic scaffolding proteins. Their nanoscale dimensions make them quite amenable for cellular uptake, while their ability to be functionalized with peptides and nucleic acids make them very appealing to drug delivery applications. Part of our ongoing work is synthesis and characterization of these particles, as well as engineering them and assessing their delivery to live cells. Nanodiscs can also incorporate integral membrane proteins. Therefore, we are also interested in developing functional biomaterials, such as immobilized protein substrates. These types of substrates can be used as diagnostic devices for drug screening and perhaps even have applications in Adoptive T Cell Therapy for cancer treatment. 