
Research Overview
The Hamm-Alvarez laboratory is primarily focused on researching the lacrimal gland in health and disease. The function of this gland is the production and release of the aqueous component of the tear fluid rich in proteins and electrolytes. This aqueous layer, together with mucin-rich proteins released from cells at the surface of the eye and lipids released by glands in the eyelids, forms the tear film. The tears are important for the health of the ocular surface as they provide nutrition, lubrication and protection from infections and foreign objects. Release of tear proteins by the lacrimal gland occurs via exocytosis, a process by which contents of secretory vesicles are released to the exterior of the cell through fusion of the secretory vesicle with the plasma membrane of the lacrimal gland acinar cell. This complex process involves multiple effectors which are a major subject of study for the laboratory.

Reduced functioning of the lacrimal gland leading to decreased production of aqueous tears leads to dry eye syndrome, which causes itchy and burning eyes and blurry vision. A severe form of dry eye is associated with Sjögren’s syndrome an autoimmune inflammatory disease that affects over 4 million people nationwide. In this disease, the lacrimal glands and the salivary glands are destroyed by the body’s own immune cells, causing severe dry eye and dry mouth. Patients with this disease may also suffer from severe systemic symptoms including fatigue and reduced kidney function, and have an increased risk of developing B-cell lymphoma. Therefore, it is important to be able to distinguish these patients from those that suffer from other forms of dry eye, and investigation of characteristic changes in the tear film are one approach to diagnosis. Intriguingly, changes in the protein composition of the tear film are associated with other diseases beyond eye diseases; the Hamm-Alvarez laboratory takes a broad approach to tear diagnostics by also studying tear biomarkers of neurological disorders such as Parkinson’s disease.
Treatment of Sjögren’s syndrome by reducing local and systemic autoimmune inflammation requires new approaches for targeting and repackaging of potent immunomodulatory agents that may have dose-limiting toxicities, enabling their safer usage, as well as identification of better therapeutic targets. The Hamm-Alvarez laboratory is engaged in both approaches.
Research Topics
- Cell Structure and Organization
- Membrane Trafficking
- Cytoskeleton
- Epithelial Cells
- Nanotechnology
- Exocytosis
- Targeted Drug Delivery
- Extracellular Matrix
- Physiology
Ongoing Projects
Ongoing research in the Hamm-Alvarez laboratory spans fundamental cell biology, to therapeutic development and drug delivery/nanoscience, to clinical investigations of tear biomarkers. Current projects include:
Project 1: Membrane Trafficking and Exocytosis in Lacrimal AciniProject 2: Tear BiomarkersProject 3: Novel Therapeutics for Treatment of Sjögren’s SyndromeProject 4: Exosomes in Lacrimal Gland Disease Biology
Project 1: Membrane Trafficking and Exocytosis in Lacrimal Acini

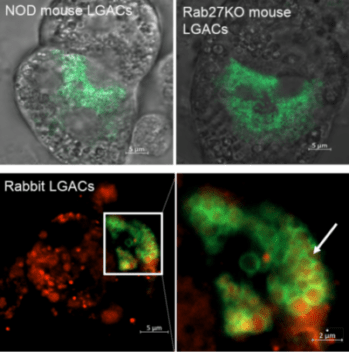
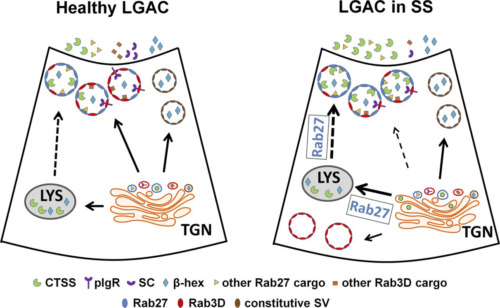
A main research focus of the Hamm-Alvarez laboratory is to explore the mechanisms involved in intracellular trafficking and release of key tear proteins, and how these processes change in diseases such as Sjögren’s Syndrome. In the healthy lacrimal gland, tear proteins are released at the apical plasma membrane of lacrimal acinar cells through constitutive mechanisms including transcytosis and through regulated exocytosis, evoked physiologically by neurotransmitters released by innervating neurons. Recently, we have focused on the role of specific Rab proteins in the exocytotic trafficking of proteins to the apical plasma membrane. Rab proteins function in diverse events in membrane trafficking including vesicle formation, trafficking along actin filaments and microtubules, and vesicle fusion. In particular, Rab3D and Rab27 isoforms (Rab27a and Rab27b) play a significant role in regulating secretion in the lacrimal gland, while changes in their abundance and localization occur in lacrimal acinar cells of a mouse model of Sjögren’s Syndrome. Our current projects involve further investigation of the role of these Rab isoforms in secretion of specific tear proteins. We are also interested in the induction of unconventional secretory pathways including secretory lysosomes and secretory autophagy by autoimmune inflammation of the lacrimal gland, and how induction of these pathways converges with those mediated by Rab3D and Rab27 isoforms. For these studies we utilize live cell imaging of lacrimal gland acinar cells transfected with fluorescent probes, as well as biochemical and molecular biological studies in Rab knockout and Sjögren’s Syndrome mouse models.
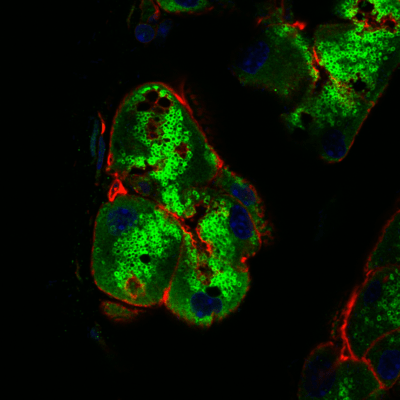
The cysteine protease cathepsin S (CTSS) is elevated in lacrimal gland and tears of a mouse model of Sjögren’s Syndrome, and in Sjögren’s Syndrome patients. We are particularly interested in exploring cytokine-induced changes in its expression, sorting and exocytosis, as well as the consequences of its increased activity on the ocular surface and in the tissue interstitium in disease.
Project 2: Tear Biomarkers

Characteristic changes in the composition of the tear film, including changes in the spectrum of tear proteins, is associated with the development of the autoimmune inflammation characteristic of Sjögren’s Syndrome. As Sjögren’s Syndrome is difficult to diagnose, often taking up to four years, with diagnosis based on clinical signs and symptoms that may not be specific to the disease, better diagnostic methods are needed for earlier and more accurage diagnosis. Our laboratory has found that the activity of Cathepsin S (CTSS), a lysosomal protease, which plays several important roles in driving autoimmune inflammation, is elevated in the lacrimal glands and tears in a mouse model of Sjögren’s Syndrome, suggesting that tear CTSS may be a potential disease biomarker of SS. We were able to confirm this potential in a clinical study at USC, showing that tear CTSS activity is increased in SS patients compared with patients with other autoimmune diseases including Rheumatoid Arthritis and Systemic Lupus Erythematosis, with other forms of dry eye and with healthy controls. We are currently investigating the utility of CTSS in combination with additional tear proteins for diagnosis of Sjögren’s Syndrome.
The tear film may exhibit characteristic changes in other diseases. Our ongoing study is exploring the potential of characteristic changes in specific proteins in the tear film to distinguish patients with Parkinson’s disease from healthy controls. As Parkinson’s disease is also difficult to diagnose, particularly prior to the appearance of motor symptoms which characterize the more advanced disease, such an approach could aid in patient diagnosis.
Project 3: Novel Therapeutics for Treatment of Sjögren’s Syndrome
The Hamm-Alvarez laboratory is focused on developing novel therapeutics for the management of lacrimal gland inflammation and ocular surface symptoms associate with Sjögren’s Syndrome and other diseases of the ocular surface. One approach, conducted in collaboration with Dr. J. Andrew MacKay in the USC School of Pharmacy, utilizes Elastin-like Polypeptides (ELPs), tunable polymers inspired by repetitive hydrophobic motifs of tropoelastin which can assemble into biodegradable and biocompatible nanoparticles. An important aspect of ELPs is their ability to form coacervates at a specific temperature known as the critical transition temperature (a function of variables including concentration, molecular weight, and hydrophobicity unique to each polymer). This genetically-engineered precision, excellent biocompatibility and environmental-responsiveness make ELPs an ideal drug carrier.
Using ELPs, we have developed novel tools for systemic and local delivery of commonly-used immunomodulatory agents including rapamycin and cyclosporine A. Our findings have demonstrated that ELP carriers can significantly modify the pharmacokinetic profile of rapamycin, consequently reducing its dose-limiting toxicity while maintaining its efficacy and potency. In addition to immunomodulatory agents, the delivery of functional regulatory peptides with ELP carriers is also being investigated in our lab to restore natural basal tearing mechanism and the integrity of the ocular surface. Finally, we are engineering targeting ligands into ELP carriers to better achieve accumulation of carried drug at sites of inflammation.
In additional studies, we are exploring the mechanisms of rapamycin in particular in treatment of the autoimmune dacryoadenitis associated with Sjögren’s Syndrome, utilizing its delivery through multiple venues including topical, intravenous and subcutaneous administration.

Project 4: Exosomes in Lacrimal Gland Disease Biology
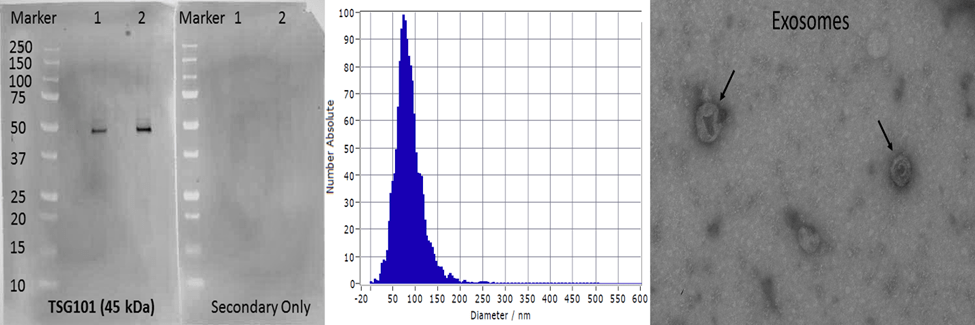
Exosomes are cell-derived vesicles between 50-120 nm in diameter with specialized functions including intercellular signaling and regulation of inflammation. Various studies indicate a potential use of exosomes in biomarker discovery. Our current projects involve the isolation, validation and biochemical and molecular characterization of exosomal contents from blood and tears for biomarker discovery in different ocular and neurological disease models.
Funding
- NIH/NEI- R01: Microtubule-Based Transport in Lacrimal Gland Function
- NIH/NEI- R01: Protein-Polymer Nanomedicine for Sjogren’s Syndrome
- NIH/NIDDK- P30: Cell and Tissue Imaging Core
- Michael J. Fox Foundation: Identification of Tear Biomarkers for Individuals with Parkinson’s Disease