Clinical History
A 58-year-old male was referred to ENT clinic due to facial nerve paralysis for about 10 months. He stated that he had gradual onset of right facial paralysis about 10 months prior to presentation that was not improved. He also had noticed a mass in the right pre-auricular region 6 months before presentation that had continuously grown since. He endorsed 40lb weight loss over 6 months. On physical examination he had a 3-4cm right firm parotid mass as well as a right level II cervical lymphadenopathy. There were no pre-auricular masses or lymphadenopathy.
Initial Work Up
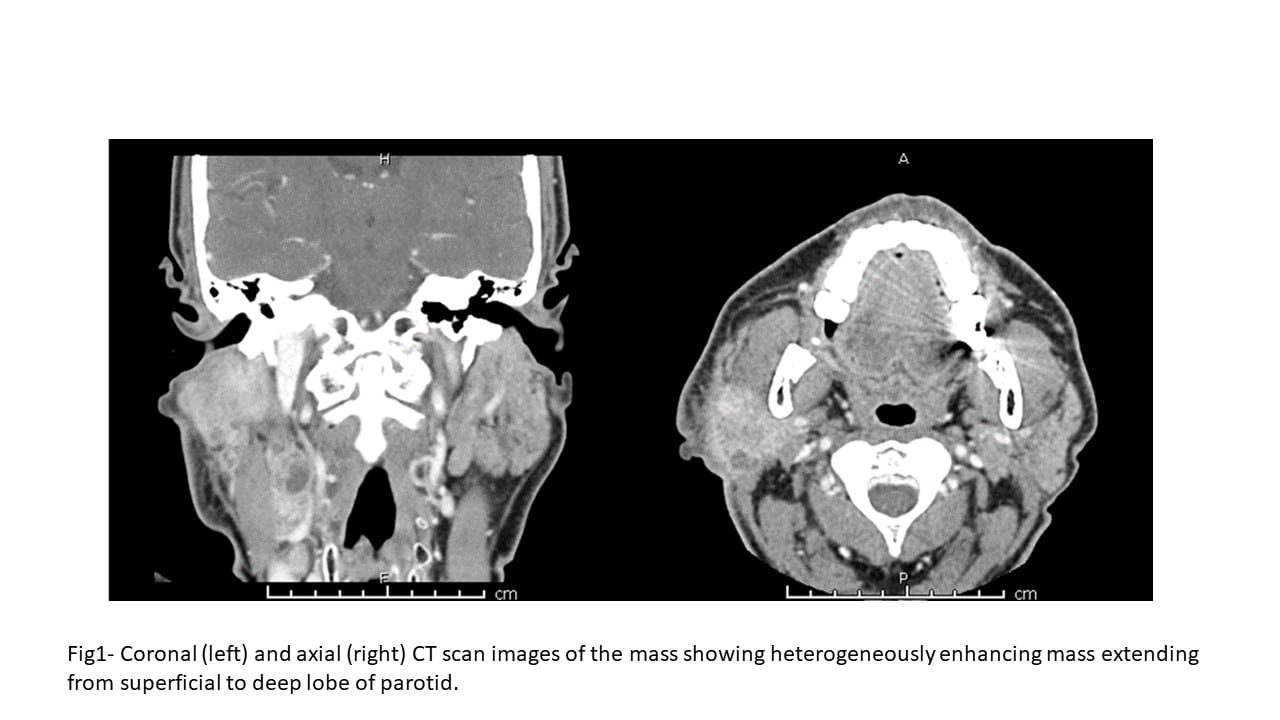
The neck CT scan with contrast showed a large bulky ill-defined heterogeneously enhancing mass measuring 4.7 x 4.2 x 4.2 cm, associated with the superficial lobe of the parotid gland extending medially behind the ramus of the mandible into the deep lobe of the parotid. A portion of the mass was extending to the level of the skin in the postauricular region. The mass appeared to invade the posterior aspect of the masseter muscle. The adjacent right cervical lymph nodes were pathologically enlarged.
Fine needle aspiration of the mass performed in the FNA clinic.
Cytologic Findings
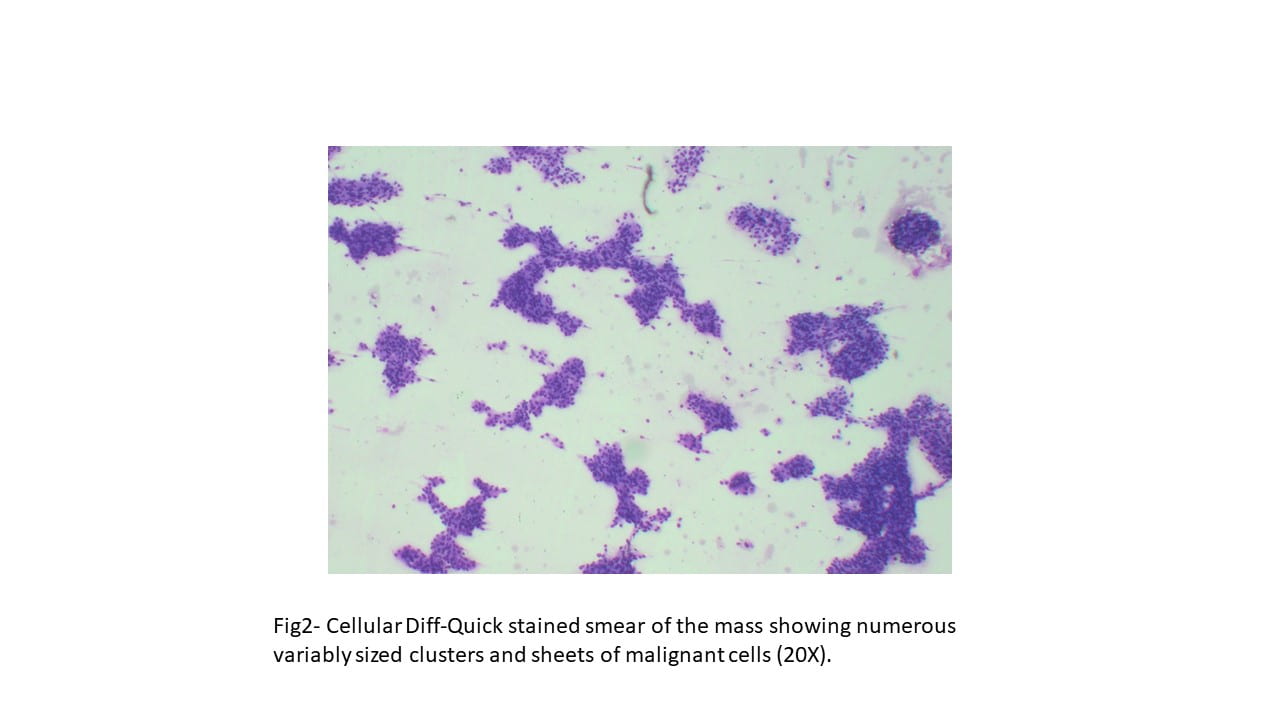
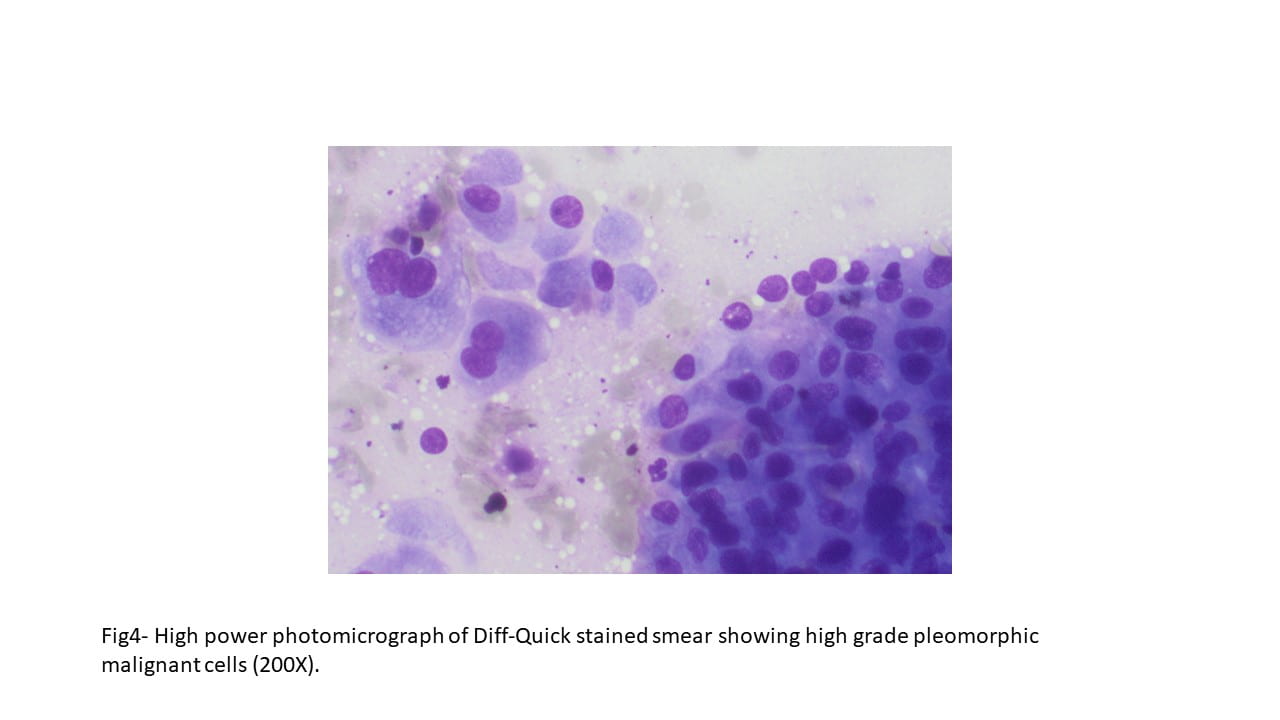
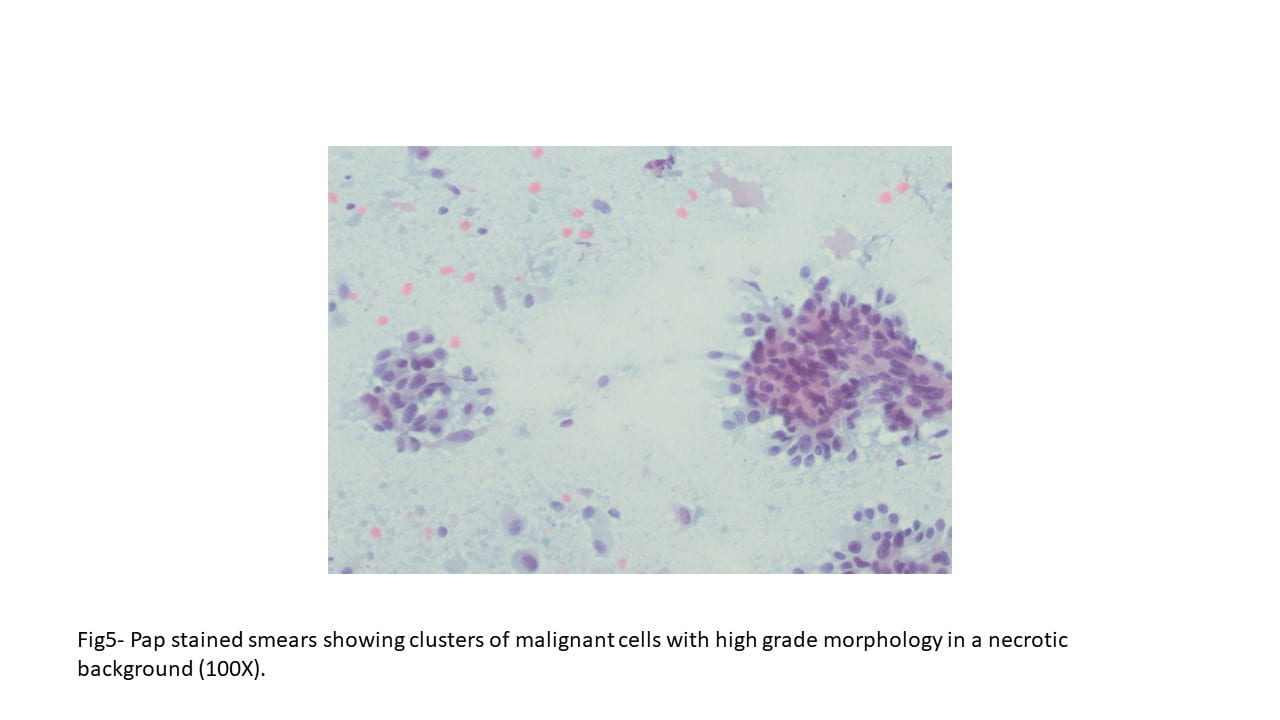
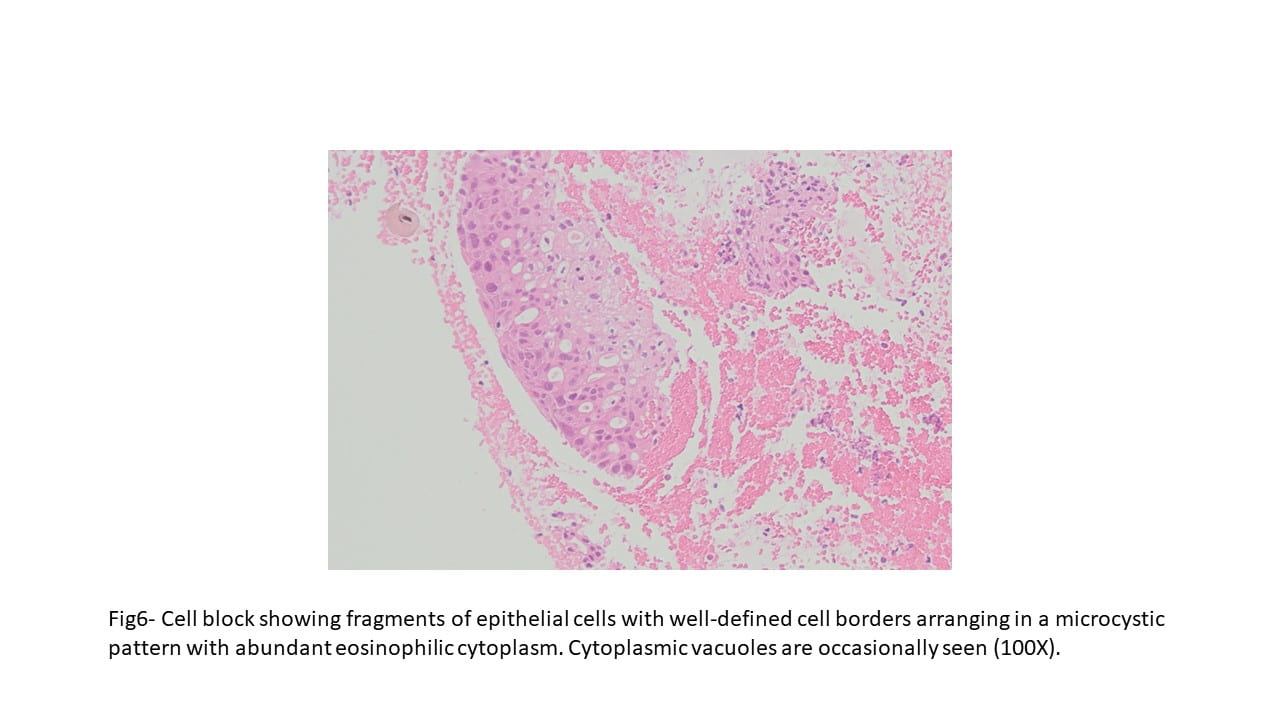
Diff-Quick and Pap-stained smears showed numerous variably sized clusters and sheets of markedly atypical cells displaying pleomorphism, hyperchromatic nuclei with irregular nuclear contours, occasional conspicuous nucleoli, moderate amount of cytoplasm, and occasional well defined cellular membranes. Necrotic debris and blood elements as well as extracellular proteinaceous material were seen in the background. The cell block showed variably sized fragments of epithelial cells with well-defined cell borders, focally arranging in a microcystic to cribriform pattern. Cytoplasmic vacuoles were occasionally seen. Mitotic figures were readily identified.

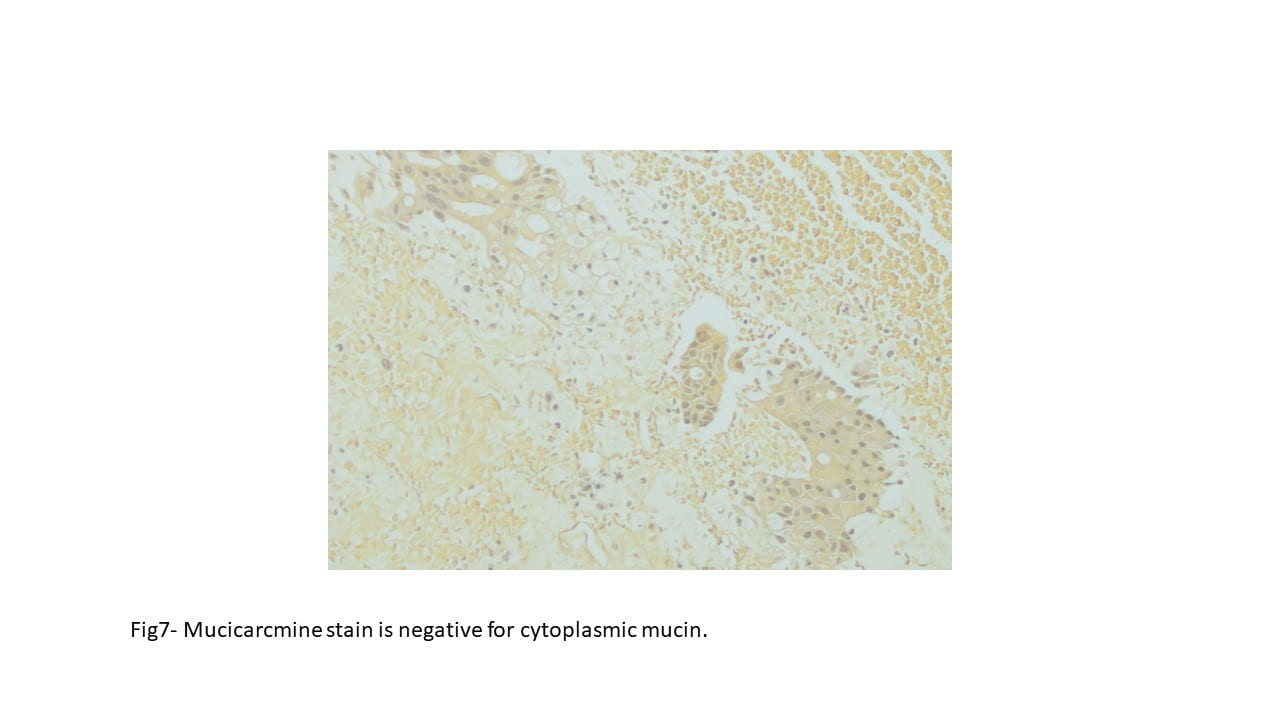
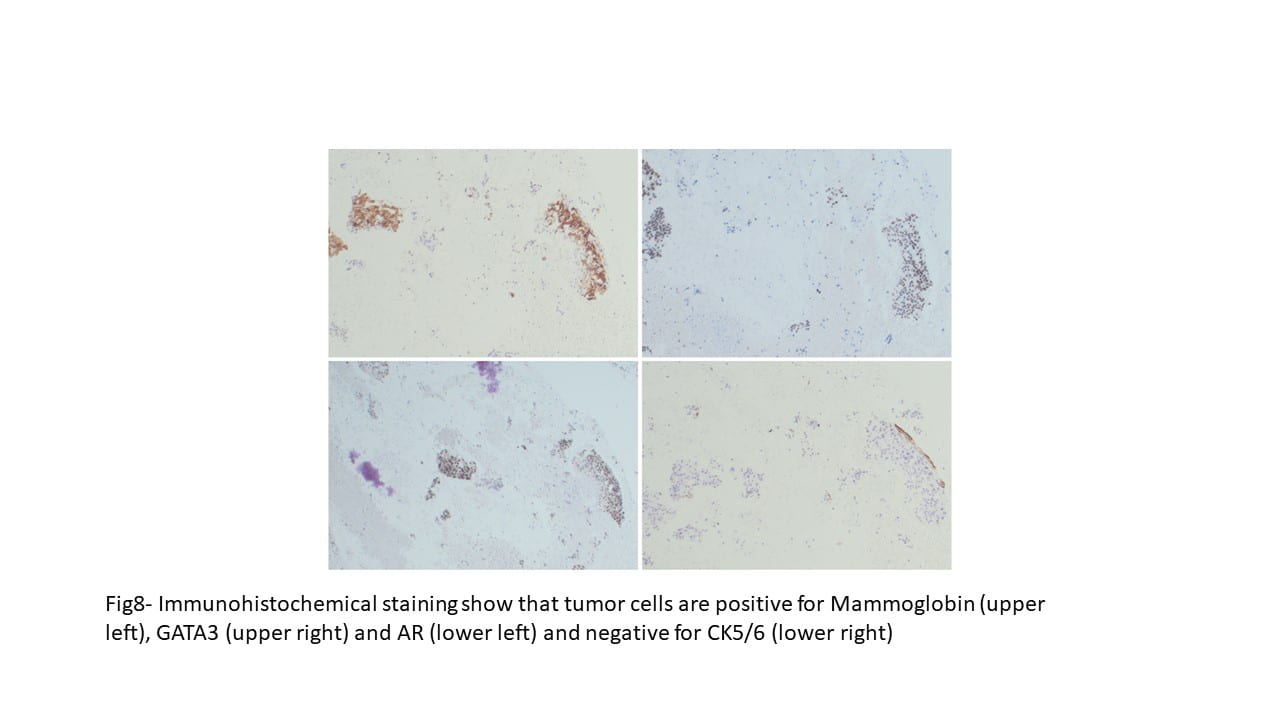

Immunohistochemical staining were performed on the cell block to further characterize the lesion. CK5/6, S100, DOG1 and p63 were negative on tumor cells, while Mammaglobin, GATA3, and Androgen receptor showed positive staining. Special staining for Mucicarmine was essentially negative.
Final Cytologic diagnosis
The overall immunohistochemical pattern of staining and morphological features were consistent with a salivary duct carcinoma. Focal presence of magenta color extracellular proteinaceous material was suggestive of a carcinoma ex-pleomorphic adenoma with salivary duct carcinoma as carcinoma component, although a distinct component of pleomorphic adenoma could not be identified.
Surgical management
The patient underwent radical parotidectomy with right levels I-V modified radical neck dissection.
Surgical Pathology Findings
On parotidectomy specimen the tumor shows an infiltrative pattern consisting of island, trabeculae, and sheets of malignant cells, some forming cribriform architectures. In areas, the solid architecture contains central necrosis (comedonecrosis) resembling a high-grade ductal carcinoma in-situ of breast. The cells display high grade morphology with pleomorphism, high N:C ratio, prominent nucleoli, moderate amount of eosinophilic cytoplasm and distinct cell borders. On the resection specimen, the tumor cells were negative for CK5/6 and p63, however, they were positive for CK7, AE1/AE3 and EMA. HER2 showed a 2+ membrane staining (utilizing breast cancer grading criteria). p53 immunohistochemical staining showed a wild type pattern of expression.
Treatment
After surgery, the patient underwent 60Gy radiation therapy. At the time of this case presentation, he is under close surveillance and the plan is to consider adjuvant systemic treatment if the tumor recurs. Since the tumor is positive for androgen receptor (AR) androgen deprivation therapy is offered to him as well. If the tumor is positive for HER-2 overexpression on FISH, anti-HER2 therapy will also be considered as a potential treatment.
Discussion
Salivary duct carcinoma is a high grade malignant salivary gland tumor which constitute approximately 10% of all malignant salivary gland tumors. At least half of salivary duct carcinomas arise as carcinoma ex-pleomorphic adenoma. It usually occurs in older individuals with peak incidence in 6th or 7th decade and are much more common in men. The parotid gland is the most common site (80%). This tumor usually presents as a rapidly growing mass with symptoms of nerve involvement. They are usually large and show an infiltrative growth pattern with foci of necrosis resembling mammary ductal carcinoma. Regional or distant metastasis may be present at time of presentation. The patients are usually managed with radical surgery with ipsilateral neck dissection followed by adjuvant radiation therapy.
On cytology, salivary duct carcinoma presents as a cellular aspirate consisting of three-dimensional crowded and cribriform groups of cells with overt malignant features displayed as medium to large polygonal cells with well-defined cell borders and abundant eosinophilic cytoplasm. Frequent mitosis and necrotic background are usually seen. The most common high-grade tumors that are among the differential diagnosis with salivary duct carcinoma, high grade mucoepidermoid carcinoma, oncocytic carcinoma and metastatic carcinoma from the breast, prostate, or lung. To distinguish these tumors, a panel of immunohistochemical stains is usually required. Salivary duct carcinoma is positive for androgen receptor (AR), whereas expression of ER and PR is rare. GATA 3 is usually positive, and most cases are also positive for GCDFP-15. HER2/neu expression is usually seen but diffuse strong membranous staining or HER2 amplification on FISH is seen in only 25% of cases. The tumors that arise as carcinoma ex-pleomorphic adenoma may overexpress translocation proteins PLAG1 or HMGA2 and more likely to express HER2.
References
- William C. Faquin, Esther Diana Rossi, Zubair Baloch, Güliz A. Barkan, Maria P. Foschini, Daniel F.I. Kurtycz, Marc Pusztaszeri, Philippe Vielh. The Milan System for Reporting Salivary Gland Cytopathology. Springer International Publishing AG 2018. Chapter 7. ISBN 978-3-319-71284-0.
- Edmund S. Cibas, Barbara S. Ducatman. Diagnostic Principles and Clinical Correlates, 5th Edition. Chapter 11. ISBN 978-0-323-63636-0
