CLINICAL HISTORY:
The patient is 65-year-old male with no past medical history who presented with anorexia and intermittent back and abdominal pain for few weeks. No other symptoms, such as nausea or vomiting, were reported. Physical examination did not reveal any specific findings. There was no family history of gastrointestinal diseases. The computed tomography (CT) scans showed 3.4 cm infiltrative pancreatic uncinate mass. No other masses or metastasis were observed on the scan. The patient was scheduled for endoscopy to perform endoscopic ultrasound fine-needle aspiration (EUS-FNA) to the mass.
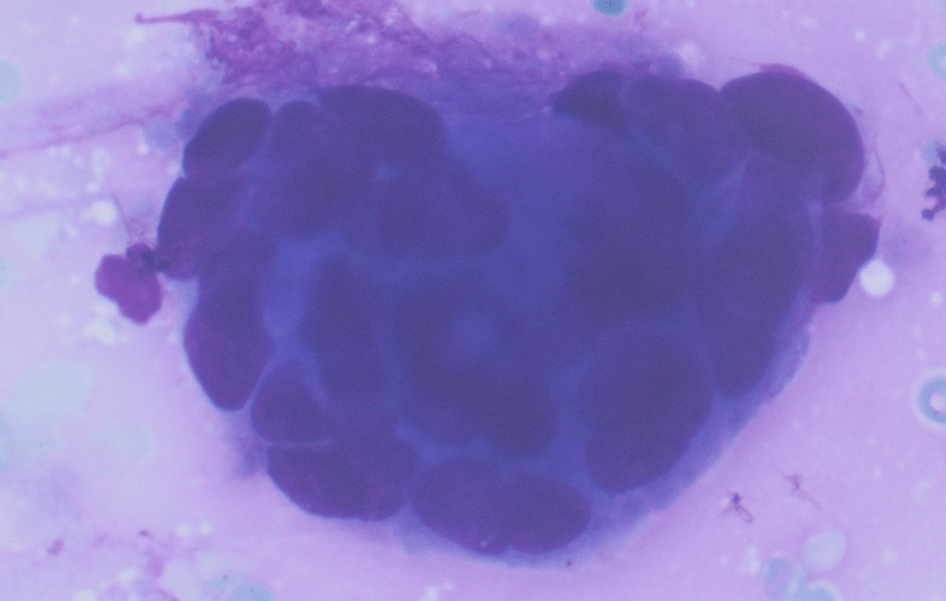
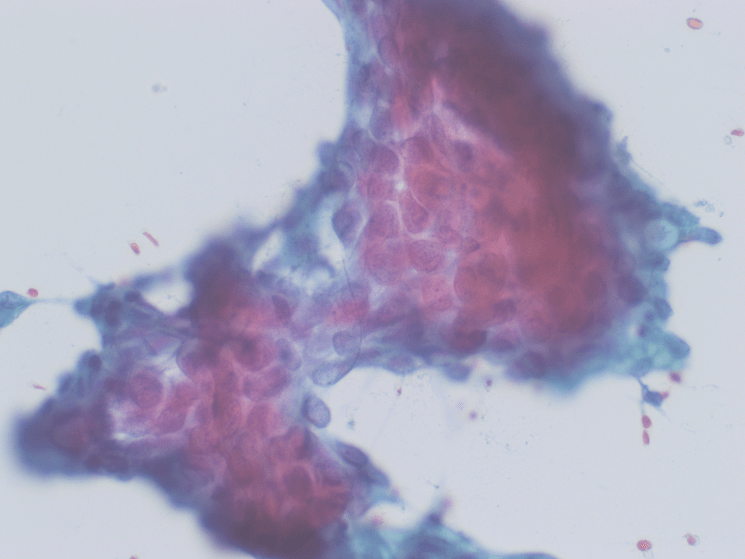
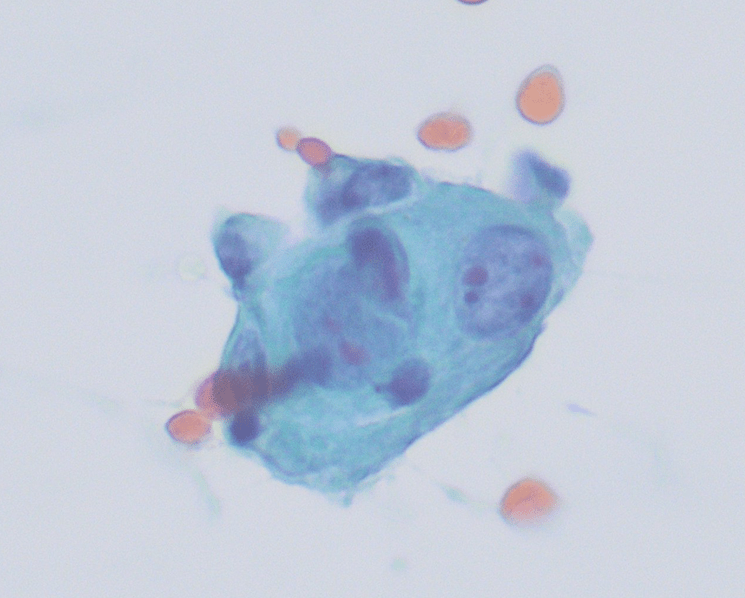
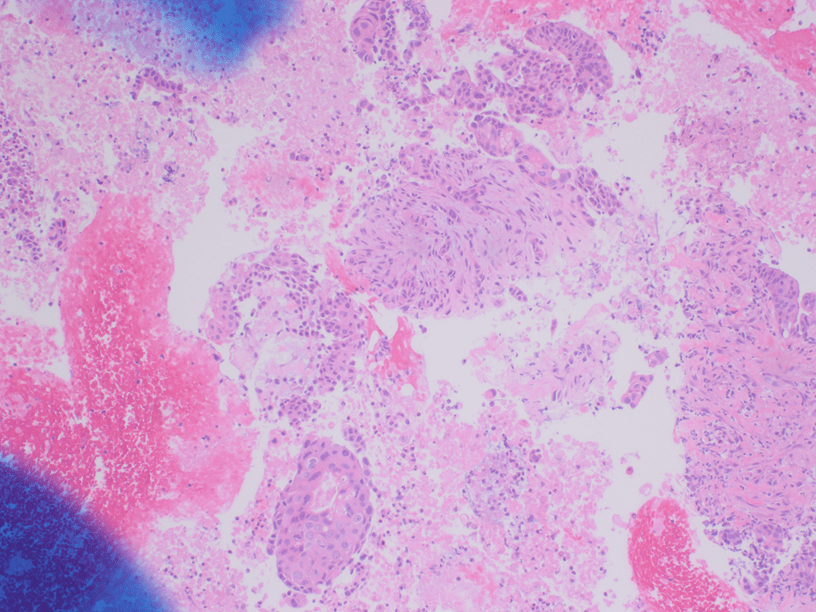
The Diff-Quik and Pap-stained smear preparations were cellular, showing Irregularly sized and shaped cohesive cluster and singly discohesive, dispersed cells. The clusters showed uneven distribution of cells. The cells showed irregular nuclear contours, nuclear enlargement, hypochromasia and hyperchromasia, anisonucleosis (greater than 4:1 variation in diameter within a single cluster), irregular chromatin distribution, and focal vacuolated cytoplasm. Few clusters showed focal dense blue cytoplasm in Diff-Quik and blue to red dense cytplasim in Pap stain. The H&E sections from cell block showed clusters with dense pink cytoplasm intimately associated with disorganized angulated glands and single cells in desmoplastic and myxoid stroma. The cells in H&E show similar cytomorphologic findings to smear preparations.
The initial differential diagnosis was pancreatic ductal adenocarcinoma (PDAC) & its variants/subtypes, reactive/reparative atypia, chronic pancreatitis, radiation changes, and metastatic carcinoma.
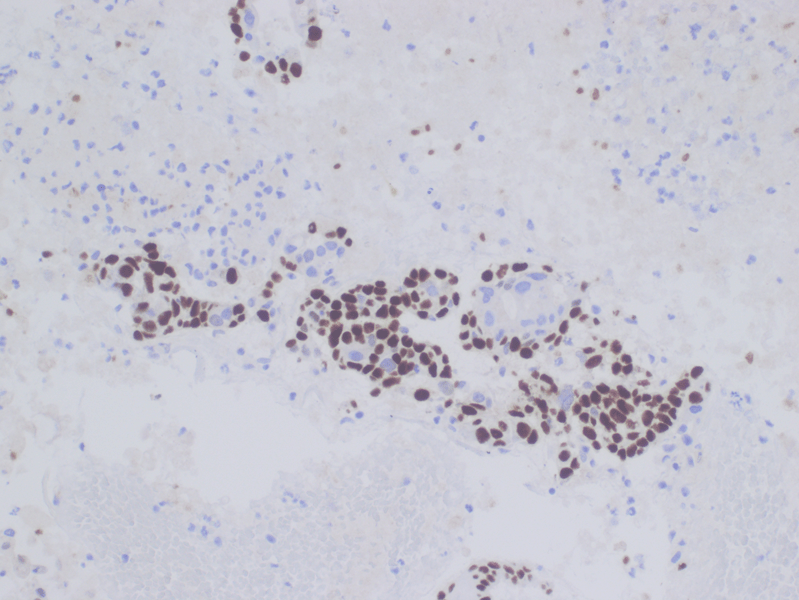
Immunohistochemical stains were performed and showed positive for CK7, p63 (focal, see fig 5), and CK20 (rare cells). The cells were negative for TTF-1, SATB2, and CDX2.
Diagnosis:
The histomorphology and immunohistochemical findings fit Adenocarcinoma with focal area of squamous differentiation consistent with adenosquamous carcinoma of the pancreas.
The patient underwent pancreaticoduodenectomy (Whipple procedure). The resection showed similar histology to cell block H&E and was ultimately diagnosed as Adenosquamous carcinoma of the pancreas.
Discussion:
Pancreatic adenosquamous carcinoma is a rare aggressive subtype of ductal adenocarcinoma and accounts for 1-4% of all exocrine malignancies of the pancreas. Also referred to as mucoepidermoid carcinoma and adenoacanthoma in older literature, adenosquamous carcinoma demonstrates both malignant squamous cell and glandular differentiation. In surgical pathology and per WHO 5ed, the squamous component arbitrarily should account for ≥ 30% of the neoplasm in order for it to qualify as adenosquamous. Squamous metaplasia of the pancreatic ductal epithelium occurs most commonly in the setting of chronic pancreatitis but is noted in the adjacent ducts of only about 4% of adenocarcinomas. Squamous metaplasia of pre-existing adenocarcinoma has been suggested by some authors as a mechanism underlying the histogenesis of pancreatic adenosquamous carcinoma. Making a diagnosis of adenosquamous carcinoma on fine needle aspiration before surgery can be difficult and it is possible when aspirates show evidence of both squamous and glandular differentiation. Adenosquamous carcinoma also may represent a metastasis to the pancreas arising from lung, colon, esophagus, salivary glands, and female reproductive organs. Pure squamous cell carcinomas of pancreas are exceedingly rare and findings of malignant squamous cells in FNA should prompt the pathologist to search for glandular differentiation for adenosquamous carcinoma. The adenosquamous carcinoma could be recognized/suggested in cytology by unique cytological features that recapitulate the histology. The squamous component often expresses p63, p40, and low-molecular-weight cytokeratins. Almost all cases harbour KRAS mutations at codon 12, and they show highly enriched TP53 mutations, along with 3p loss and mutations in the RNA surveillance gene UPF1. Immunohistochemically, they show loss of p16 (CDKN2A) protein expression, loss of SMAD4 (DPC4) protein, and strong nuclear p53 immunoreactivity, which is similar to the molecular signature found in Pancreatic ductal adenocarcinomas (PDACs). Adenosquamous carcinomas appear to have a worse prognosis than pure ductal adenocarcinomas even when resected, with a median survival time of about 9 months. The presence of any squamous component in the neoplasm appears to portend a worse prognosis.
REFERENCES:
- WHO Classification of Tumours Editorial Board. Digestive system tumours. Lyon (France): International Agency for Research on Cancer; 2019. (WHO classification of tumours series, 5th ed.; vol. 1). https://publications.iarc.fr/579.
- Skafida E, Grammatoglou X, Glava C, Zissis D, Paschalidis N, Katsamagkou E, Firfiris N, Vasilakaki T. Adenosquamous carcinoma of the pancreas: a case report. Cases J. 2010 Feb 1;3:41. doi: 10.1186/1757-1626-3-41. PMID: 20205828; PMCID: PMC2825199.
- Cibas ES, Ducatman BS. Cytology: Diagnostic Principles and Clinical Correlates. Philadelphia, PA: Elsevier; 2021.
- Paramythiotis, D., Kyriakidis, F., Karlafti, E. et al. Adenosquamous carcinoma of the pancreas: two case reports and review of the literature. J Med Case Reports 16, 395 (2022). https://doi.org/10.1186/s13256-022-03610-5.
- Aşkın OÇ, Adsay NV. Adenosquamous carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/pancreasadenosquamous.html. Accessed March 25th, 2023.
