The information on this page was prepared by:
| Megan Page | mpage@usc.edu | Computer Engineering |
| Laveena Rajan | lrajan@usc.edu | Chemical Engineering |
Natural Gas: Introduction
Natural gas is the world’s cleanest burning fuel. From the depths of the world’s oceans, natural gas, which is composed primarily of methane, hydrogen sulfide, ethane, butane, pentane and propane, is by far one of the most reliable sources of energy. The carbon and hydrogen components of natural gas are thought to have been produced by the remains of microscopic marine organisms that were deposited at the bottom of the seas and oceans. These organic materials, after having remained buried under layers and layers of other sediments for long periods of time are then transformed under high temperatures and pressure into natural gas. From the marine shale in which it was deposited, natural gas is then squeezed out into porous sedimentary rocks. Migrating upward through the porous rock, natural gas is then captured by drilling a hole into the reservoir rock. The figure below recounts visually the steps mentioned above.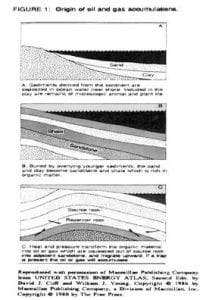
In the United States alone, natural gas provides for 24 percent of the total energy consumption, and an estimated 55 percent of American homes use natural gas for home heating. Through an intricately woven 1.3 million-mile network of underground pipe, natural gas is delivered to about 175 million American consumers. Not to mention that natural gas production in the United States accounts for 24 percent of the world’s total natural gas production. Measured in British Thermal Units, or BTU, natural gas is the primary supplier of energy for many important machines we use today: stoves, ovens, cars, and many more.