The information on this page was prepared by:
| Yi Luo | yiluo@usc.edu | Electrical Engineering |
| Ryan Dumouchelle | dumouche@usc.edu | Computer Engineering and Computer Science |
| Joanne Zhang | joannezh@usc.edu | Civil Engineering |
Molten Carbonate Fuel Cells
Compared to the other forms of fuel cells, molten carbonate fuel cells operate at much higher temperatures and consequently achieve a much higher fuel-to-electricity use efficiency. Additionally, molten carbonate fuel cells eliminate the need for external processors to isolate the hydrogen for utilization. (1)
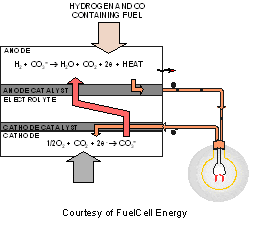
www.nfcrc.uci.edu/fcresources/FCexplained/images/fcTypeIMAGES/moltencarbonate.gif
The fuel cell itself consists of a minimal number of parts as can be seen in Figure 1 (2)The two electrodes that instigate the fuel cell contain numerous small pores that allow permeability. Inside the fuel cell are two different catalysts that allow for two different processes to occur. These catalyst react differently with natural gas that acts as the fuel for this fuel cell. When natural gas is the fuel, methane (the main ingredient of natural gas) and steam are converted into a hydrogen-rich gas inside the fuel cell stack (a process called “internal reforming”) (1). This is a unique principle to molten carbonate fuel cells. Additionally, since this fuel cell operates at such high temperatures, multiple fuel types can be used. At the anode, hydrogen reacts with the carbonate ions (CO3) to produce water, carbon dioxide, and electrons.) Specifically, the electrode contains multiple alkali metals (potassium, lithium, and sodium salts) that act as a conductor of the produced electrons. In order for this process to occur, the electrode must be heated to around 600 to 700 degrees Celsius to produce the alkali metals into a molten state (2). In this molten state, the metals act as a conductor for ions. This molten state conducts the electrons passing through. The electrons travel through an external circuit, creating electricity, and returning to the cathode. There, oxygen from the air and carbon dioxide recycled from the anode react with the electrons to form CO3 ions that replenish the electrode and transfer current through the fuel cell, completing the circuit (1).
Sources:
- http://www.fe.doe.gov/programs/powersystems/fuelcells/fuelcells_moltencarb.shtml
- http://www.nfcrc.uci.edu/fcresources/FCexplained/FC_Types.htm