The information on this page was prepared by:
| Curtis Wong | curtiswo@usc.edu | Electrical Engineering |
| Steven Michael Yee | syee@usc.edu | Biomedical Engineering |
Direct Methanol Fuel Cell (DRMFC) and Proton Exchange Membrane Fuel Cell (PEMFC)
The environmental and societal implications of proton exchange membrane fuel cells (PEMFC) and direct methanol fuel cells (DMFC) are potentially greater than any current source of energy that is presently in widespread use. The greatest promise is in the emissions that would be reduced. There are many culprits of today’s pollution, chief among them nitrogen oxides, sulfur oxides, carbon monoxide, and the many types of hydrocarbons. These fuel cells have essentially no emissions of these pollutants. An additional bonus is that there is extremely low noise production when the fuel cells are in use.
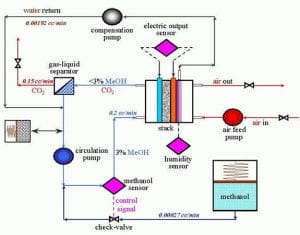
While it is true that the PEMFC variant is more efficient, it does require hydrocarbon fuels to be reformed to create a supply of pure hydrogen gas that must then be safely stored. The DMFC variant, while less efficient, does not require pure hydrogen as the catalyst on its anode draws hydrogen straight from liquid methanol (CH3OH), which is also known as methyl alcohol. The DMFC variant does produce carbon dioxide, but this could be turned into an advantage if the carbon dioxide could be stored. This potential advantage exists because when hydrogen gas is reacted with carbon dioxide, methanol is produced, the very fuel that the DMFC requires (Figure 5).
This potential could very turn out to be vital for future energy concerns. It is estimated by David Stipp that approximately 94% of all crude oil on the planet has already been discovered (Stipp, David. “The Coming Hydrogen Economy.” Fortune. 21 October 2002 www.fortune.com). In addition to a finite supply of crude oil, one must consider the ever growing population of the planet. It is expected by Jeremy Rifkin that the world population will increase from 6.2 billion to 7.5 billion by just the year 2020 (Rifkin, Jeremy. The Hydrogen Economy: The Creation of the Worldwide Energy Web and the Redistribution of Power on Earth. New York : Putnam Publishing Group, 2002.). The ever growing rate that which our world population grows will obviously place constantly increasing demands on oil. A point will be reached where oil cannot be supplied at the rate needed and/or will be exceeding expensive to recover. It is estimated by the United States Geology Society (USGS) that 20.7 billion barrels of recoverable oil are in existence. However, it is also estimated that only 7.7 billion barrels can be recovered with existing technology. Also with current technology, Rifkin approximates that only 3 billion barrels can be recovered at a rate of two dollars per barrel. It seems obvious that increased research into making fuel cells become an economical choice for energy is needed.
Figure 7
http://auto.howstuffworks.com/hy-wire.htm
The possible applications for fuel cells seem as numerous as one can imagine. Both stationary and mobile applications of all sizes are envisioned. The most widely talked about application are the numerous vehicles under development that utilize fuel cells. Among them are the Toyota FCHV, GM Hy Wire,(Figure 7) and Daimler Chrysler F-Cell. In addition to the companies just mentioned, almost every major automotive company is involved in research concerning the application of fuel cells in vehicles. The application of fuel cells in vehicles shows especially great promise. The efficiency of most internal combustion engines range between 15 and 18 percent. The potential of fuel cells being considered for vehicles usually range from 35 to 55 percent efficient. This would lead to an increase in the average miles per gallon of vehicles from 23.8 of today to approximately 55 (How Stuff Works. 20 October 2003 . <www.howstuffworks.com/fuel-cell4.htm>). There are numerous possible stationary applications as well, ranging from power generation facilities for homes and businesses to marine applications on offshore oil and underwater facilities. Furthermore, DMFC applications involving small portable devices such as PDAs, cell phones, TVs, and laptops are being explored. More research is required to tap into the massive potential of fuel cells and their tremendous environmental and societal benefits.
Sources:
1. http://www.nfcrc.uci.edu/
2. http://www.ballard.com/
3. Rifkin, Jeremy. The Hydrogen Economy: The Creation of the Worldwide Energy Web and the Redistribution of Power on Earth. New York: Putnam Publishing Group, 2002.
4. Stipp, David. “The Coming Hydrogen Economy.” Fortune. 21 October 2002 . www.fortune.com
5. http://www.howstuffworks.com/fuel-cell4.htm