The information on this page was prepared by:
| Yi Luo | yiluo@usc.edu | Electrical Engineering |
| Ryan Dumouchelle | dumouche@usc.edu | Computer Engineering and Computer Science |
| Joanne Zhang | joannezh@usc.edu | Civil Engineering |
Solid Oxide Fuel Cells
Solid Oxide Fuel Cells are very different from all the various types of fuel cells. First of all, all aspects of the fuel cell are composed of all solid-state materials (the anode, cathode, and electrolyte are composed of ceramic-like substances) (1). A negative aspect to this is the possibility of the cell cracking under extreme conditions (2). Secondly, they operate at very high temperatures. The Solid Oxide Fuel cell operates anywhere between 600 to 1000 degrees Celsius. The ceramic nature of the fuel cell allows for these temperatures to occur(1) Usually the efficiency of this type of fuel cell is around 60%. However, if the waste heat is utilized in a particular manner that would incorporate it back into the fuel cell stack, efficiency can reach anywhere up to 85%. At this efficiency this fuel cell can output up to 100kW of electricity. (2) Finally, this particular type of fuel cell can be utilized in a tubular fashion that allows for an even greater electrical output (See Figure 1)(3)
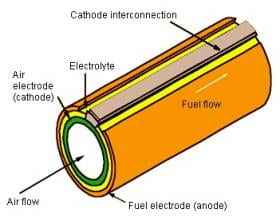
www.csa.com/hottopics/Fuecel/Gifs/image006.jpg
The fuel cell consists of a minimal number of parts, as compared to the other fuel cells. Although a variety of oxide combinations have been used for solid oxide electrolytes, the most common to date has been a mixture of zirconium oxide and calcium oxide. Formed as a crystal lattice, the hard ceramic electrolyte is coated on both sides with specialized porous electrode materials (1). This is the most common combination for a solid oxide fuel cell. Initially, a gas consisting of hydrogen gas and carbon monoxide is required for this fuel cell. This fuel will react and initiate the electro-chemical reaction. The ceramic anode and cathode are porous, allowing for the hydrogen and oxygen molecules (respectively) to permeate through to the electrode (See Figure 2) (4) The hydrogen molecules permeate through to the electrode (which contains oxygen gas) where it will react and form water. The electrons resulting from this reaction will consequently pass through an external wire and create an electrical flow. The excess oxygen gas will permeate through the cathode where it will meet up with the utilized electrons. At this point, it will be released leaving oxygen gas and heat (resulting from the reaction) as the only excess products.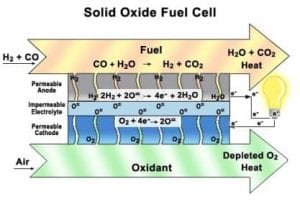 www.csa.com/hottopics/Fuecel/Gifs/image002.jpg
www.csa.com/hottopics/Fuecel/Gifs/image002.jpg
Sources:
1)http://www.fe.doe.gov/programs/powersystems/fuelcells/fuelcells_solidoxide.shtml ( 17 Oct 2003 )
2) http://www.iit.edu/~smart/garrear/fuelcells.htm ( 17 Oct 2003 )
3) http://www.fuelcells.org/fctypes.htm ( 18 Oct 2003 )
4) http://www.csa.com/hottopics/Fuecel/oview.html