The information on this page was prepared by:
| Matthew Boatman | boatman@usc.edu | Computer Engineering and Computer Science |
Introduction
Fuel cells offer an exceptional alternative to the other forms of energy generation presented on this website. Proton Exchange Membrane Fuel Cells in particular are coming closer to a reality as innovations are made making them cheaper and more efficient. Only a few years ago these fuel cells were far too expensive for consumer use and were unacceptable means of energy conversion.
Before we advance our discussion we should clarify the role of fuel cells in energy production. Fuel cells are less of a method of energy production as they are a method of energy conversion like a battery. The catalytic reaction required to obtain the energy from the hydrogen is efficient but still not economical enough to actually produce energy it simply converts it. The “Hydrogen Economy” proposed by industry pundits is still dependent on solar power, or other alternative energy sources to be truly “green”. Fuel Cells are faced with other technical and political problems as well such as finding readily accessible sources of hydrogen, a problem which has possibly been solved by another type of fuel cell called Direct Methanol invented here at the University of Southern California , and obtaining industry wide adoption of fuel cells for use in vehicles and homes.
A fuel cell is a very simple electrochemical device. The chemical reaction powering a hydrogen fuel cell is exactly the same as the burning of hydrogen. The reaction is:
Anode: H ↔ 2H+ + 2e-
Cathode: O4H+ + 4e- ↔ 2H2O
A fuel cell instead of burning hydrogen to create light and heat energy produces electrical energy. A fuel cell (Figure 1) consists of an electrolyte, usually platinum, sandwiched between two electrodes dubbed the anode(negative) and cathode(cathode). The fuel, usually hydrogen, is passed over the anode which often includes the catalyst thus ionizing it. Ionization separates the electrons from their nuclei creating positively charged hydrogen ions and negatively charged, freely roaming, electrons. The electrons with no bonded nucleus must find a way to reunite with their positive nuclei. The freely roaming electrons pass around the electrolyte constitutes, a wire, producing an electric current.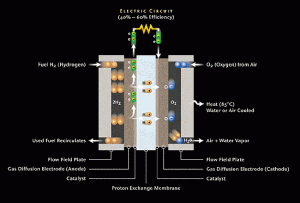
What delineates the different types of fuel cells is really the material used for the electrolyte and the fuel used. In the case of the Proton Exchange Membrane the electrolyte consists of a thin plastic sheet allowing hydrogen ions to pass through it. The membrane is coated on both sides with highly dispersed platinum alloy particles (The Online Fuel Cell Information Center || TYPES OF FUEL CELLS). The electrolyte used is a solid organic polymer poly-perfluorosulfonic acid. The solid electrolyte has the advantage of being corrosion resistant and small enough to fit inside vehicles and possibly small portable devices.
Numbers of these individual fuel cell assemblies are connected in series with a plate containing channels for the two reactants, hydrogen and oxygen, and channels to to siphon off wastewater wedged between each cell. One of the inherent difficulties with fuel cells is the larger the fuel cell the less efficient it becomes the solution to this is of course the fuel cell stack which combines many small fuel cells into one large power producing unit.